INTRODUCTION
Chronic lateral and medial elbow tendinopathy, commonly referred to as tennis elbow and golfer’s elbow, respectively, are painful conditions affecting athletes and non-athletes alike. Elbow tendinopathy has been estimated to occur in 1–3% of the population, and is the most commonly diagnosed musculoskeletal condition of the elbow (Herquelot et al. 2013; Sanders et al. 2015; Shiri et al. 2006). Men and women are equally affected, and patients are typically between 35 and 54 years of age (De Smedt et al. 2007; Haahr and Andersen 2003b, 2003a; Kwapisz et al. 2018; Le et al. 2018).
The pathology of elbow tendinopathy was initially thought to be due to an inflammatory process, and until recently, the condition was commonly referred to as epicondylitis. While it may start as an inflammatory condition in the acute stages, the chronic condition is typically associated with tendon degeneration resulting from repeat overloading and abnormal microvascular responses (Shiri et al. 2006; De Smedt et al. 2007; Barnes, Beckley, and Smith 2015). Bunata et al. described the histology of chronic elbow tendinopathy as consisting of disorderly tendon fibers in combination with fibroblasts and atypical vascular granulation-like tissue, focal hyaline degeneration, and calcific debris surrounded by hypercellular and degenerative tissues (Bunata, Brown, and Capelo 2007).
Several conservative treatment options have been described to treat epicondylitis, including rest, activity modification, nonsteroidal anti-inflammatory drugs (NSAIDs), bracing, and physical therapy. When conventional therapy is ineffective, extracorporeal shock wave therapy and ultrasound-guided interventions, including steroid injections, needle tenotomy, and more recently, the use of autologous blood (AB), platelet-rich plasma (PRP), or tissue products of amniotic origin, have been used (Bunata, Brown, and Capelo 2007; Le et al. 2018; Arirachakaran et al. 2016; Mattie et al. 2017; Boden et al. 2019; Gosens et al. 2011; Mishra et al. 2014; Peerbooms et al. 2010; Stenhouse, Sookur, and Watson 2013; Thanasas et al. 2011). Despite the many treatment options, there is continued debate on the most appropriate and effective treatment for chronic elbow tendinopathy. It is widely reported that 10-15% of tendinopathy patients fail conservative treatment (Sanders et al. 2015; De Smedt et al. 2007; Burn et al. 2018; Nirschl and Ashman 2003; Titchener et al. 2013; Walker-Bone et al. 2012), and many patients fail multiple treatment options. However, despite an increased understanding of the differences in tendon pathology between acute inflammatory tendinitis and chronic degenerative tendinosis, the treatment algorithms are typically the same for both disease states.
Therefore, this study was conducted to test the hypothesis that ultrasound-guided, minimally invasive tenotomy using the TenJet resection device (HydroCision Inc., North Billerica, MA) might provide symptomatic pain relief and functional recovery to patients with chronic degenerative tendinosis.
METHODS
Study design
This prospective, non-randomized, multicenter study was conducted at 3 private practice centers by 4 physicians specializing in non-operative orthopedic care and the use of ultrasound imaging for diagnosis and image-guided interventions. The study was reviewed and approved by the Western Institutional Review Board (protocol number 20161401).
Patients were eligible to participate in the study if they met the following criteria: greater than 18 years of age; chronic lateral or medial elbow pain for greater than 3 months; clinical history and examination consistent with lateral or medial elbow tendinosis; sonographic or magnetic resonance imaging evidence of medial or lateral elbow tendinosis as evidenced by tendon thickening and hypoechogenicity, with or without hypervascularity on Doppler examination, with or without cortical irregularities, with or without intrasubstance tear; at least 1 instance of non-operative treatment of steroid injections, physical therapy, injection of PRP or other biologic formulations, or needle tenotomy; and a willingness to provide informed consent to participate in the study with the required follow-up.
Patients were excluded from the study if they had documented ipsilateral upper extremity musculoskeletal conditions unrelated to elbow tendinosis, had bleeding disorders, were on anti-coagulant medication, or had an active local or systemic infection.
The outcome measures for the study included the patient-rated elbow evaluation (PREE) questionnaire scores for pain and function, visual analog scale (VAS) scores for pain, procedure-related complications, and adverse events. Baseline, preoperative PREE, and VAS assessments were collected during patient enrollment. Post-procedure follow-up assessments were collected at 2 and 6 weeks and at 3, 6, and 12 months. The PREE and VAS measures were administered by the nursing staff.
The PREE questionnaire is a validated instrument consisting of 20 items for 2 subscales (pain and disability), with responses for each item rated on a 0–10 numeric scale; higher scores represent more severe pain and disability. A total score out of 100 is computed by equally weighing the pain score (sum of 5 items) and the disability score (sum of 15 items, divided by 3). The PREE evaluation has been found to have a high intraclass correlation coefficient of 0.95 and high construct validity when compared to established questionnaires, such as the American Shoulder and Elbow Surgeons-Elbow scoring system (Spearman’s rho ~ 0.92) (Vincent et al. 2013).
Minimally invasive tenotomy and debridement technique using the TenJet device
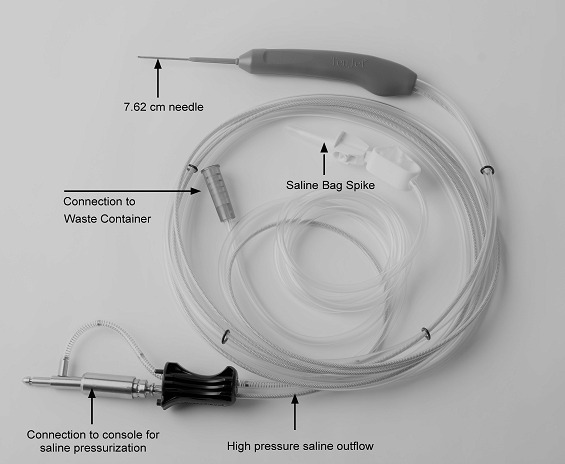
The TenJet device was designed to enable selective resection and removal of degenerative tendinopathic tissue without causing harm to the adjacent healthy tendon using high-pressure saline jet as a resection tool. The device (Figure 1) consists of a 7.62 cm (3 inches), 12-gauge needle with 2 lumens: 1 that delivers high-velocity sterile saline with pressures up to 14,000 psi to the needle tip, and the other that evacuates resected tissue and waste fluid. The pressure and velocity of the saline create their own suction due to the Venturi effect at the needle tip, effectively pulling degenerative tissue into a 1.65 mm cutting window where the saline jet acts as a cutting blade, and the suction evacuates cut tissue. The device is connected to a console via a pump cartridge that pressurizes the sterile saline. The TenJet device and console have been cleared by the United States Food and Drug Administration for the cutting and removal of soft and hard tissue in minimally invasive, open, or arthroscopic orthopedic procedures. HydroCision’s hydrosurgery technology has been used safely and effectively for over 2 decades in various minimally invasive resection procedures, including wound tissue debridement and spinal disc nucleus removal (Cristante et al. 2016; Han, Kim, Park, et al. 2009; Vaisman and Ordia 2016).
The procedures were performed in an ambulatory surgery center or hospital outpatient setting. Depending on patient and physician preference, the procedure was performed using a local anesthetic, conscious sedation, or general intravenous anesthesia. Ultrasound imaging was utilized to identify areas of pathologic tendon tissue prior to the procedure. The entire area was prepped to create a sterile field, and under ultrasound guidance, a local anesthetic was injected to create a skin wheel and then administered down to the pathologic tendon tissue. Once adequate anesthesia was confirmed, a stab incision was made, and the scalpel was directed to the previously identified pathology, sometimes through the tendon sheath, creating a path for the treatment device. The TenJet needle tip was advanced to the pathology under ultrasound guidance, and the foot pedal was depressed to initiate the flow of the high-pressure saline jet. Under continuous ultrasound guidance, the device was maneuvered to expose the cutting window at the needle tip to the pathologic tissue. The procedure was performed until the area of tendon tissue that was identified with ultrasound imaging was deemed to have been adequately treated using observation of visual changes on ultrasound (less hypoechoic tissue) and tactile changes with the device (less tissue resistance). The incisions were closed with Steri-Strips™ (3M™, St. Paul, MN, USA). No sutures were required.
Use of post-procedural nonsteroidal anti-inflammatory medications was discouraged but permitted if necessary. The first follow-up visit was at 2 weeks for a wound check and the initiation of an exercise program that focused on eccentric exercises of the common flexor or extensor tendons of the elbow in the operative limb. It was recommended that patients restrict lifting with the operative limb to loads less than 4.5 kg (10 pounds) for 6 weeks. At the 6-week post-procedure visit, activity was progressed as pain allowed.
Statistical analysis
This was a single treatment arm study designed to evaluate clinical outcomes with the TenJet resection device. Descriptive statistics are used to describe the overall safety and clinical performance of the device. Continuous variables are presented as means, standard deviations, and ranges. VAS and PREE scores are reported as the mean difference from baseline, standard deviations at each time point, and as a percentage of change from baseline at each follow-up time point.
A repeated-measures analysis of variance (ANOVA) was used to test the hypothesis that minimally invasive, ultrasound-guided tenotomy using the TenJet resection device provides symptomatic pain relief and functional recovery for patients with chronic medial and lateral epicondylitis. An ANOVA was also applied to the VAS and PREE scores. Tukey’s post-hoc test was applied to identify significant differences between baseline and each of the follow-up time points.
RESULTS
Demographics and clinical history
Twenty-nine patients (10 male, 19 female) with a mean age of 48 years (range 33–65 years) were treated with a minimally invasive tenotomy using the TenJet resection device between August 2016 and March 2018 (Table 1). The mean height and weight were 158.75 cm (range 157.48–190.5 cm) and 83.73 kg (54.43–147.87 kg), respectively. The mean body mass index (BMI) was 29.8 kg/m2. The mean duration of symptoms before the procedure was 26.1 months (range 4–96 months); over 70% of the patients had experienced symptoms for longer than 1 year.
All patients had documented failed conservative treatment. Twenty-seven (93.1%) patients had tried a minimum of 2 conservative treatments; 16 (55.2%) had completed at least 6 weeks of physical therapy, 21 (72.4%) had tried using elbow straps, and 22 (75.9%) had attempted activity modification.
Twenty-three (79.3%) patients reported having received a corticosteroid injection as a prior treatment. Only 1 (3.4%) patient reported not having a corticosteroid injection, and 5 (17.2%) did not report whether or not they had received a steroid injection. Six (20.7%) patients, representing 8 (25.0%) elbows, had received 3 or more steroid injections.
No patient reported receiving prior PRP or stem cell injections, elbow surgery, or having any concurrent significant medical conditions.
Procedure and complications
The ultrasound-guided, minimally invasive tenotomy procedure was performed in 32 elbows (29 patients), including 26 (81.25%) with lateral pathology, 4 (12.50%) with medial pathology, and 2 (6.25%) with both medial and lateral pathology (Table 1). Three patients received treatment on both the left and right elbows, 2 of whom received treatment on both elbows on the same day. Conscious sedation was administered in 21 (72.4%) patients, while 6 (20.7%) received a local anesthetic, and 2 (6.9%) received general anesthesia. The mean total procedure duration, defined as the time from the initial stab incision to the removal of the handpiece, was 12 min. There were no device malfunctions or complications related to the procedure (Table 2).
Follow-up
Twenty-five (86.2%) patients (27 elbows, 84.4%) completed the 12-month follow-up visit. Two (6.9%) patients (3 elbows, 9.4%) were lost to follow-up, and 2 (6.9 %) patients (2 elbows, 6.2%) withdrew from the study after being referred for open debridement.
Of the 2 patients referred for open debridement, 1 was referred at the 2-week post-procedure follow-up due to pain, and the other was referred after the 3-month appointment due to a worsening of symptoms. The patient with worsening symptoms was non-compliant to the post-procedure protocol, having resumed bowling between the 2- and 6-week appointments, aggravating their lateral elbow symptoms without discrete injury. At the 3-month follow-up, the patient’s pain and function scores had significantly worsened and were 66% higher than those at baseline.
Clinical outcomes at follow-up
The mean difference in the total PREE and VAS scores from baseline improved significantly at 2 weeks and continued to improve through 12 months (both, p < 0.05; Table 3).
Analysis of individual PREE pain items associated with rest, lifting a heavy object, and performing a repeat activity also revealed statistically significant improvement from baseline to each follow-up time point (p < 0.05; Table 4).
DISCUSSION
The treatment of chronic tendinosis using conservative treatment options remains a challenge for many physicians, impacting a patient’s ability to be pain-free during activities of daily living, participate in recreational or professional sports, or engage in work that involves physical labor. Surgical debridement of degenerative tendon tissue has long been the standard of care to treat degenerative tendon pathology once all conservative or minimally invasive treatment options have failed (Burn et al. 2018; Nirschl and Ashman 2003; Kroslak and Murrell 2018; Vinod and Ross 2015). However, procedure numbers remain low, perhaps due to the reluctance of patients to opt for surgery until they have exhausted all possible conservative treatment options.
In this study, the mean duration of symptoms was 26.4 months, with 70% of patients reporting chronic pain symptoms that had persisted for over 1 year; 93.1% had tried a minimum of 2 conservative treatments. These statistics highlight the need for additional treatment options for chronic tendinosis.
Several studies have reported on patients with chronic epicondylitis who were treated with needle tenotomy, needle tenotomy with PRP, or percutaneous ultrasonic tenotomy (Boden et al. 2019; Mishra et al. 2014; Stenhouse, Sookur, and Watson 2013), the 3 common treatment choices after corticosteroid injections or physical therapy.
Mishra et al. reported on a randomized controlled trial of 230 patients treated with percutaneous needle tenotomy with or without leukocyte-rich PRP. At 12 weeks, patients receiving needle tenotomy with PRP reported a 55.1% improvement in their VAS pain scores compared to the 47.4% improvement in the needle tenotomy group (p = 0.163).
Stenhouse, Sookur, and Watson reported on a prospective randomized controlled trial of 28 patients treated with percutaneous needle tenotomy or percutaneous needle tenotomy with leukocyte-poor PRP. At 6 months, the VAS pain scores improved 34% in the needle tenotomy group compared to 48.5% in the needle tenotomy with PRP group (p = 0.74).
In 2019, Boden et al. reported on a retrospective study comparing outcomes in patients treated with PRP injections (32 patients) versus those treated with percutaneous ultrasonic tenotomy (30 patients). In an average follow-up of 17 months in the PRP group and 10 months in the percutaneous ultrasonic tenotomy group, they found improvements of 45.2% (p = 0.0051) and 60% (p = 0.0005), respectively.
By contrast, in this study the average improvement in VAS pain scores was 72.1% at the 3-month follow-up (p < 0.05), 72.9% at the 6-month follow-up (p < 0.05), and 73.9% at the 12-month follow-up (p < 0.05).
Although the mechanism of tendon healing after any of the above-mentioned treatments or treatment with the study device is not yet clearly understood, the preliminary positive outcomes observed in this study could be attributed to the ability of the study device to remove the abnormal degenerative tissue within the tendon, similar to surgical debridement techniques, which neither needle tenotomy nor biologic injections are recognized to do.
Unexpectedly, we observed an apparent difference in outcomes in patients with a clinical history of 3 or more corticosteroid injections. When stratified by the number of cortisone injections, those who had received 3 or more previous injections had worse post-procedure improvement in PREE outcomes than those receiving fewer than 3 cortisone injections (Figure 2). However, those patients with 3 or more injections also had a higher average duration of symptoms (41.0 months vs. 21.8 months) and worse VAS and PREE scores at baseline compared to those with fewer than 3 injections (VAS, 5.5 vs. 4.9; PREE 67.9 vs. 55.4), possibly indicating a more severe disease. Since only 6 patients in this study had 3 or more cortisone injections prior to the procedure, a statistical comparison could not be made. The effects of corticosteroids on tendon cells and the risk factors associated with 3 or more corticosteroid injections in tendon treatments have been previously documented in the literature (Degen et al. 2017; Zhang, Keenan, and Wang 2013; Puzzitiello et al. 2020). Additional studies would need to be performed to further understand whether the results observed in patients receiving 3 or more corticosteroid injections in this study were due to more severe disease or to the impact of corticosteroids on tendon health and its ability to repair.
Limitations of the study include its relatively small sample size and single-arm, observational design, which lacked a control group and randomization. While randomizing patients with long-standing periods of chronic symptoms who have failed multiple conservative treatments, to another conservative treatment can be difficult, there might be future opportunities to develop protocols based on tendon pathology rather than the duration of symptoms or failed treatments and allow for randomization and cross-over between treatment arms. Additional information such as cause of injury, time to return to sports or work, grading of tendon pathology could also be evaluated to better understand the efficacy of this treatment option in varied patient populations.
Overall, the clinical outcomes from this study provide information that medical providers may use to help guide further research into the clinical utility of this treatment option for patients presenting with chronic tendinopathy in the elbow. With the availability of in-office diagnostic ultrasound imaging, physicians now have an opportunity to evaluate and classify underlying tendon pathology during a clinical exam. These findings could help guide treatment choices, and the device used in the study could be offered as a potential treatment option for patients presenting with chronic degenerative tendinosis.
CONCLUSIONS
Ultrasound-guided, minimally invasive tenotomy using the TenJet resection device provided symptomatic pain relief and functional recovery to patients with chronic tennis or golfer’s elbow. Additional studies to further validate these outcomes in a larger cohort of patients, understand the effect of corticosteroids on the duration of chronic symptoms on tendon healing, and the impact on the return to sports or return to work in specific patient populations may be necessary.
Data availability statement
De-identified participant data are available upon reasonable request from the authors (ORCID identifier: 0000-0002-8950-8921).
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
COMPETING INTERESTS
RK reports non-financial support from HydroCision Inc. during the conduct of the study and personal fees, and others from HydroCision Inc., outside the submitted work.
CV reports non-financial support from HydroCision Inc. during the conduct of the study and personal fees from HydroCision Inc. outside the submitted work.
FUNDING
The authors did not receive a grant for this research from any funding agency in the public, commercial or not-for-profit sectors.